Enhancing Brain Tumor Segmentation using Berkeley Wavelet Transformation and Improved SVM
Abstract
Aims
This research gives insight into the various machine learning models like enhanced Support Vector Machines (SVM), Convolutional Neural Networks (CNN), Recurrent Neural Networks (RNN), and Artificial Neural Networks (ANN) in brain tumor recognition by medical imaging. This research provides an accurate model for allowing a better form of diagnostic method in neuro-oncology, with the help of precision, recall, and F1-score metrics. The present study, therefore, also provides a basis on which further predictive models for medical image analysis can be developed.
Background
This study is premised on the critical need for improved diagnostic tools within medical imaging in the fight against the prevalence of brain tumors. A model showing meaningful performance in the practices of brain tumor detection includes enhanced SVM, CNN, RNN, and ANN. The models have been evaluated based on their accuracy, precision, recall, and F1 score to investigate their performance and potential. Consequently, the models addressing the subject of neuro-oncological diagnostics were evaluated.
Objective
This study seeks to critically evaluate the performance of four different machine learning models: enhanced SVM, CNN, RNN, and ANN, in detecting a brain tumor. It will be determined from this study which model has the highest accuracy, precision, and recall in finding a brain tumor. It will then lead to the improvement of diagnostic techniques in neuro-oncology.
Methods
The methodology of this research involved a detailed assessment of four machine learning models: enhanced SVM, CNN, RNN, and ANN. Each model was evaluated based on accuracy, precision, recall, and F1 score metrics. The analysis focused on their ability to detect brain tumors from medical imaging data, examining the models' performance in identifying complex patterns within varied feature spaces.
Results
The outcome of this study reveals that the enhanced Support Vector Machine (SVM) model performed the highest compared to the other models, demonstrating an impressive 97.6% accuracy. In the case of CNN, it achieved 95.76% for effectively identifying hierarchical features. The RNN showed a good accuracy of 92.3%, which was pretty adequate for sequential data treatment. The ANN achieved a high accuracy of 88.77%. These findings describe the differences and strengths of both models and have possible applications in brain tumor detection.
Conclusion
This study conclusively established how much potential emerged for machine learning models to improve the detection capabilities of brain tumors. Addressing a performance perspective, the enhanced SVM ranked first. Again, this is proof of its critical importance as a tool in accurate diagnostic medicine. Based on these findings, further development of machine learning techniques in neuro-oncology will lead to an increase in diagnostic accuracy and treatment outcomes. It lays the fundamental foundation for betterment in any predictive model to be made in the future.
1. INTRODUCTION
1.1. The Significance of Brain Tumor Segmentation in Medical Imaging
Since brain tumor segmentation is essential to the accurate diagnosis and treatment of neurological disorders, it is a highly significant field in medical imaging. With the increasing incidence of brain tumors worldwide, there is a requirement for solid segmentation strategies to improve medical diagnosis and treatment recommendations with increasing precision [1-3]. For the planning of treatment, monitoring of progression, and response-to-treatment assessment, a clinician must be able to delimit the boundaries of a tumor from the surrounding healthy tissues. Brain tumor segmentation represents one of the prerequisites for radiologists and oncologists during medical imaging, where magnetic resonance imaging prevails. Accurate segmentation recovers spatial and morphological information regarding the complete position of the tumor, which further assists medical professionals in describing the form, dimensions, and placement of cancer within the complex structure of the human brain. Whatever the best course of treatment may be, chemotherapy, radiation therapy, surgery, or a combination of these, this information is crucial [4, 5].
Furthermore, the advancement of personalized medicine depends on precise segmentation. No less important is that better and more accurate segmentation allows for more effective and patient specific treatment regimes, individualized to the characteristics of each patient's tumor. This translates easily into two ways in which patient outcomes are improved: all around better quality of life for the treated individual and fewer unnecessary interventions. Researching the natural history of brain tumors and evaluating the efficacy of new treatments in light of studies and clinical trials also require accurate segmentation. Researchers employ robust segmentation techniques to monitor the evolution of tumor morphology and size over time. These findings offer crucial information about how the disease progressed and how well the treatment worked [6-8].
1.2. Motivation and Research Objectives
Techniques such as Berkeley Wavelet Transformations and Support Vector Machines (SVM) were developed to enhance the process of medical image analysis used for brain tumor segmentation with more accurate effectiveness and better results [9, 10]. These techniques could help; however, quite often, the traditional method fails to detect the subtlety among all the major types of brain tumors. The Berkeley wavelet transform offers a persuasive solution in facilitating the extraction of coarse and fine-grain image features and allows multi-level analysis. In the case of medical imaging, it becomes helpful in situations where primitive differences like the tissue could help to recognize the presence of pathological conditions [11, 12].
The optimization target is in line with refining the performance of the classifier algorithm SVM, a robust model applied in a broad sense in the domain of medical image analysis. Though generally highly respected for their capability in binary classification, the problem related to brain tumor segmentation should be taken as more complicated and handled on a more sophisticated and nuanced level. Improving the capability of SVM to detect complex and small changes, particularly in tumor region delineation, must be prioritized to make the process more precise and reliable. High sensitivity, noise, and unpredictability are essential elements of medical images, and the SVM must be tailored to meet their specific requirements [13, 14].
This work's main goal is to combine the benefits of Berkeley Wavelet Transformation with an improved SVM to optimize the brain tumor segmentation process. These cutting-edge techniques aim to enhance diagnosis and treatment planning in the long run by more precisely and consistently segmenting brain tumors.
2. LITERATURE REVIEW
Reliable identification of brain tumors carries out an important task, which impacts the imaging diagnosis and overall treatment of the medical field. Many segmentation techniques to improve the accuracy of tumor localization have been looked into by researchers. The inherent complexity of medical image data has just made the traditional methods, which often rely on manual intervention and basic thresholding techniques, inappropriate in dealing with them. However, these breakthroughs have brought a shift in the trend toward more and more complicated techniques, like wavelet-based transformations and machine learning. Among the most critical applications in medical image analysis lies the application of Berkeley Wavelet Transformation (BWT) [15, 16].
It makes multi-resolution analysis and feature extraction relatively easy through a more flexible tool originating under wavelet theory, the Berkeley Wavelet Transformation. BWT has been proven to be a compelling method for discovering the slightest texture, intensity, and spatial relationships in medical image segmentation for brain tumors. Several studies show that multi-resolution characteristics of BWT enhance the accuracy of segmentation along the boundary of the tumor. Furthermore, it is very flexible and can handle data heterogeneity in medical imaging; thus, it can be readily applied to various modalities, including CT and MRI scans [17-19]. This combination has been demonstrated to be useful in applications involving machine learning in the medical imaging domain. The enhanced SVM is an enhancement over the conventional SVM and has been increasingly used in diagnosing brain tumors.
SVMs are very good at binary classification tasks, so they can be used to distinguish tumor from non-tumor regions in medical images. SVM can function more effectively if it is tailored and optimized to match the requirements of medical imaging data. Moreover, the literature emphasizes the integration of different machine learning models, such as Convolutional Neural Networks (CNN), Recurrent Neural Networks (RNN), and Artificial Neural Networks (ANN), in an attempt to achieve more accurate and automated brain tumor detection [20, 21].
The use of machine learning-based methods to categorize brain tumors has increased during the last several years. CNNs have shown promising results in recognizing spatial relationships within medical image data. The ability of CNNs to automatically learn hierarchical features sets them apart. The accuracy of tumor localization is improved by CNNs' spatial perception capability. Furthermore, RNNs are excellent at capturing temporal variations in medical imaging data because they are designed to handle sequential dependencies. This enables scientists to completely understand how a tumor evolves over time. Comparing these state-of-the-art machine learning models for analysis yields results that are more accurate and effective than traditional segmentation methods [22, 23].
While machine learning and wavelet-based transformations have shown a lot of promise, challenges remain. Large and diverse datasets for trustworthy model training, generalization to different imaging modalities, and the interpretability of complex models all require further study. The ethical quandaries that arise from using these technologies in clinical settings and from seamlessly integrating these state-of-the-art techniques into the existing workflows in healthcare further impede wider adoption [24].
3. METHOD
3.1. Data Collection
The dataset used in this study was compiled from a wide range of medical imaging tests, including brain CT and MRI scans. The collection includes both brain tumor-free and tumor-filled images, among many other kinds of images. The suggested segmentation model must be trained and evaluated using this sizable dataset under various pathological circumstances and anatomical variations. The sample brain tumor images are demonstrated in Fig. (1).
Sample brain tumor images from The BraTS Dataset.
Thoroughly splitting the dataset into training and testing sets made it possible to evaluate the model in great detail. Seventy percent of the 1713 images in the dataset were set aside for training. Based on this large portion of the dataset, the model is able to identify complex patterns and attributes associated with both tumor and non-tumor classes. Three-quarters of the dataset, or 735 images, were set aside specifically for the trained model's testing. This section enhances the reliability of the results and simulates real-world scenarios to offer an unbiased evaluation of the model's generalization capabilities on anonymous data.
3.2. Data Source and Ethical Considerations
The medical imaging data used in this study were obtained from an open-source repository on Kaggle, a widely recognized platform for hosting datasets. The dataset, titled “The BraTS Dataset,” includes anonymized MRI and CT images specifically curated for research purposes. All data are publicly available and were accessed under the terms and conditions outlined by the repository, ensuring compliance with ethical standards. Since the dataset is open-source and does not include personally identifiable information, ethical clearance was not required for this study. The use of this dataset aligns with ethical research practices and supports reproducibility in scientific investigations.
3.3. Dataset Size and Mitigation of Bias
The dataset used in this study, obtained from an open-source Kaggle repository, includes a limited number of MRI and CT images. Since the dataset size is relatively small, we employed several measures to mitigate potential biases and enhance the robustness of our analysis:
(1) Data Augmentation: To increase the effective size of the dataset and improve its diversity, we applied data augmentation techniques such as rotation, flipping, scaling, and random cropping. These methods artificially expanded the training data, allowing the model to generalize better to unseen data and reducing the risk of overfitting.
(2) Cross-Validation: A k-fold cross-validation approach (with k=5) was implemented to ensure robust model evaluation across multiple splits of the data. This technique minimizes the influence of biases that may arise from a single training-test split and provides a more comprehensive assessment of model performance.
(3) Generalizability and Benchmarking: The dataset used is a standard benchmark dataset frequently utilized in similar research studies. While the sample size is limited, its open-source nature enables reproducibility and comparability with related work in the field of medical imaging and machine learning.
(4) Future Considerations: We acknowledge the limitations posed by the small dataset size and its potential impact on the generalizability of the results. Future studies will incorporate larger and more diverse datasets, including multi-institutional and real-world data, to further validate and refine the proposed models.
By implementing these strategies, we aimed to mitigate potential dataset biases and ensure the reliability of the results presented in this study. These efforts have been incorporated into the revised manuscript to enhance its clarity and rigor.
3.4. Pre-processing Steps
The consistency and quality of the medical imaging dataset used to segment brain tumors were improved by meticulous pre-processing. The latter is an important step that deals with intrinsic variations and artifacts in the raw CT and MRI images to attain better performance of the segmentation model. Normalization is also a simple pre-processing step, and the first pre-processing step equates the pixel intensities in every image. This normalization process kept pixel values within a fixed range for all pixels, thus minimizing the effect of intensity variation caused by changes in imaging hardware or imaging protocols. In contrast, pixel value normalization made the model more robust and not very sensitive to the changes in picture acquisition parameters.
All images were after that scaled to make sure spatial resolutions were consistent post-normalization. With the last step in place, the model would be compatible with inputs of constant size, thus ensuring a homogeneous dataset. Resizing allowed images of diversity in the training pipeline, and in the next model training, it increased the computational efficiency. Furthermore, applying noise reduction techniques increases the images' signal-to-noise ratio. Applying methods like median filtering or Gaussian blurring helped suppress extraneous details and promote a cleaner representation of anatomical structures because medical imaging data is prone to noise artifacts. In addition to improving the images' visual clarity, this noise reduction allowed for more accurate feature extraction in the later stages of the segmentation model.
By using intensity normalization techniques specific to medical imaging, potential variations in imaging modalities and intensities were taken into account. For the model to correctly identify relevant features, all pixel values, regardless of the intensity characteristics of the original image, had to be calibrated to a common scale.
Table 1 provides a brief overview of all the necessary pre-processing steps, emphasizing how each one improves, cleans, and standardizes the medical imaging dataset in order to get it ready for brain tumor segmentation.
| Pre-processing Step | Description |
|---|---|
| Normalization | Standardizing pixel intensities across all images to a consistent scale, reducing sensitivity to intensity variations. |
| Resizing | Establishing a uniform spatial resolution to ensure consistent dimensions across all images, facilitating model training. |
| Noise Reduction | Implementing techniques like Gaussian blurring or median filtering to suppress irrelevant details and enhance image clarity. |
| Intensity Normalization | Adjusting pixel values to a standardized scale, addressing variations in imaging modalities and intensities. |
3.5. Berkeley Wavelet Transformation (BWT)
Berkeley Wavelet Transformation (BWT) is utilized in this work to improve the features used in brain tumor segmentation. The powerful Berkeley Wavelet Transformation image analysis tool allows for multi-resolution decomposition and the extraction of both local and global features from an image. Brain tumors can be segmented using this technique, which also provides a more thorough analysis of the tumor boundaries by identifying minute details and patterns in the medical images.
The Berkeley Wavelet Transform uses a set of variable wavelet filters in size and in more than one direction. Mathematically, they are applied to the original image to comprehensively cover the image domain in the integral domain. The overall decomposition at each level uses wavelet decomposition iteratively to split the image into approximation and detail coefficients. In this process, the high-frequency extraction is performed with the detail coefficients, while the low-frequency information is estimated from the approximation coefficients. For now, that model can recognize the very detailed minute features in the image and the global structures by multi-resolution analysis.
The improved features obtained from the Berkeley Wavelet Transformation help to provide a more discriminative representation of tumor boundaries in the context of brain tumor segmentation. The various features used in this research are shown in Table 2. The extraction of texture information, subtle intensity variations, and spatial relationships within an image are all made possible by multi-resolution analysis. These features are required to precisely define tumor regions. The goal of the research is to maximize the segmentation model's capacity to distinguish between areas with and without tumors by incorporating these improved features into the model's later phases. In the end, this will raise the overall precision and accuracy of the segmentation results.
| Feature Category | Description |
|---|---|
| Intensity-based Features | Statistical measures (mean, standard deviation, skewness, kurtosis) of pixel intensity within the tumor region. |
| Texture-based Features | Grey-level co-occurrence matrix (GLCM) statistics, capturing spatial relationships of pixel intensities. |
| Shape-based Features | Geometric attributes such as area, perimeter, and compactness characterize the shape of segmented tumors. |
| Wavelet-based Features | Coefficients from Berkeley Wavelet Transformation capturing multi-resolution details in the image. |
| Statistical Shape Descriptors | Moments and other shape descriptors provide quantitative information about the tumor's spatial distribution. |
| Gradient-based Features | Magnitude and orientation of gradients, highlighting edges and boundaries within the segmented tumor area. |
| Spatial Location Features | Coordinates and spatial relationships of tumor centroids, aiding in understanding the tumor's position. |
3.6. Step-by-Step Description of Processes
• Data Collection: We gathered MRI scans from publicly available medical imaging datasets, focusing on both healthy and tumor-affected brain images. (Details discussed in section 3.1).
• Data Normalization: Each image was normalized to ensure uniform intensity distribution across the dataset. Normalization was performed by scaling pixel values to a range of 0 to 1, which helps in reducing the computational complexity and improving model convergence.
• Noise Reduction: To minimize the impact of noise, we applied Gaussian filtering with a kernel size of 3×3. This step enhances the clarity of the images by smoothing out the variations that do not contribute to the detection of tumors.
3.6.1. Feature Extraction Using Berkeley Wavelet Transformation (BWT)
• Wavelet Decomposition: Each MRI image was decomposed into multiple sub-bands using the Berkeley Wavelet Transformation. This involves applying a series of wavelet filters to the image, breaking it down into different frequency components.
• Selection of Wavelet Coefficients: From the decomposed sub-bands, relevant wavelet coefficients were selected based on their contribution to the differentiation between healthy and tumor tissues. This selection was made using an energy-based criterion where coefficients with higher energy were retained, as they often represent significant features.
• Dimensionality Reduction: To reduce computational load and avoid overfitting, Principal Component Analysis (PCA) was applied to the selected wavelet coefficients, retaining 95% of the variance.
3.6.2. Tumor Detection using Enhanced Support Vector Machines (SVMs)
• Kernel Selection: We used a Radial Basis Function (RBF) kernel for the SVM, which is effective for non-linear classification tasks. The RBF kernel helps to map the input features into a higher-dimensional space where a hyperplane can effectively separate the classes.
• Hyperparameter Tuning: The SVM model's hyperparameters were optimized using a grid search technique with 5-fold cross-validation. The optimal values identified were:
• C (Regularization Parameter): 1.0, which controls the trade-off between achieving a low training error and a low testing error.
• Gamma (Kernel Coefficient): 0.01, determining the influence of individual training samples and controlling the flexibility of the decision boundary.
• Training and Validation: The SVM model was trained on 80% of the dataset and validated on the remaining 20%. This split ensured that the model had sufficient data to learn while maintaining a robust evaluation.
3.7. Various Machine Learning Models used in This Research
It is strategically possible to successfully and accurately forecast brain tumors with the aid of a gigantic ensemble of machine-learning models. The conventional but enhanced SVM provides a strong foundation for effective classification through its discriminative power.However, in some research, ANN structures are also used, which are strong in identifying complex relationships and patterns. Recurrent neural networks are the best techniques for handling the temporal characteristics and sequential dependencies of medical imaging data. Furthermore, the model uses Convolutional Neural Networks (CNNs) to automatically extract hierarchical features from image data, capturing spatial relationships needed for more intricate predictions [25, 26].
3.7.1. Enhanced Support Vector Machine (SVM)
In this study, we employed an enhanced version of the Support Vector Machine (SVM) to improve the accuracy and robustness of brain tumor detection. The enhancements made to the traditional SVM model include the following key modifications:
-
Adaptive Kernel Selection:
- Unlike a standard SVM that typically uses a fixed kernel function, we implemented an adaptive kernel strategy that dynamically selects the most appropriate kernel (linear, polynomial, or radial basis function) based on the characteristics of the input data.
- The selection process involved calculating the kernel alignment score during the initial training phase to choose the kernel that maximizes the margin between classes and improves classification accuracy.
-
Feature Selection and Dimensionality Reduction:
- We integrated a feature selection mechanism that employs Recursive Feature Elimination (RFE) in conjunction with the SVM model to iteratively remove less significant features.
- Principal Component Analysis (PCA) was also applied to the selected features to reduce dimensionality while retaining 95% of the variance. This step not only minimized computational complexity but also enhanced the model’s performance by focusing on the most informative features.
-
Hyperparameter Optimization:
- The hyperparameters of the SVM, that is, the regularization parameter C and the kernel coefficient gamma, were optimized with the grid search method and combined with 5-fold cross-validation. This rigorous search strategy allowed the selection of the hyperparameters providing maximal generalization performance on the data not seen so far.
- The enhanced SVM used a regularization parameter (C) of 1.5 and a gamma value of 0.01, which were found to be optimal for this specific dataset.
-
Integration of a Weighted Cost Function:
- In our study, we use a weighted cost function to treat the class imbalance in the dataset and place a higher value on instances misclassified of the minority class; that class would be the tumor cases. This will make the model more sensitive when it comes to detecting tumors and hence reduce the risk of false negatives, a very important aspect in any medical diagnostic.
-
Custom Kernel Function Development:
- A kernel function was custom-developed, combining some of the properties of the RBF and polynomial kernels. The novel characteristic of this hybrid kernel is that it captures global tendencies besides local fluctuations within both the tumor and non-tumor classes of nonlinear data.
-
Outlier Detection and Handling:
- An outlier detection mechanism was integrated into the training process using one-class SVMs. This step was crucial for identifying and handling outliers in the dataset, which could otherwise skew the decision boundary and negatively impact model accuracy.
3.7.2. Artificial Neural Network
In this respect, artificial neural networks infer a dynamic and adaptive framework for the prediction of brain tumors as they pick up complex patterns and relationships associated with the data. An Artificial Neural Network (ANN) is a network of connected nodes or elements arranged in layers to carry out processing in the same manner as the brain. Input representations of input features can be input into the input layer and further be analyzed by a set of hidden layers and finally predict the output layer. The flexibility of ANNs makes them a good choice for the difficult task of classifying brain tumors; the latter models' complex nonlinear interactions manifest in medical imaging data. There are two phases of operation for artificial neural networks: training and inference. The network trains itself by adjusting the internal weights or parameters so that labeled examples drive down the difference between expected and actual outputs. Most of the time, artificial neural networks infer relationships and patterns from the training set using optimization algorithms. The trained artificial neural network infers, in this stage, that unknown and new data resembles what it has learned [27-29].
3.7.3. Recurrent Neural Network
Herein, it is essential to note that RNN does constitute significance for brain tumor prediction because the features it processes are sequential and temporal; medical imaging data is used. Opposing the conventional neural network, the architecture in RNN is designed to pay special attention to the received information corresponding to the previous time step, which makes them particularly suitable for sequence tasks, such as medical imaging data that exist in the form of the series and need to be processed with high accuracy. The recurrent connections between the hidden layers within the RNN architecture allow this network to remember early inputs. In a prediction task of brain tumors, a time change in some characteristics is significant for reliable classification when the network's memory can hold the sequential nature of the medical images. An RNN cannot function unless input sequences are processed repeatedly. At every time step, the network receives new input and data from the previous time step, which it uses to update its internal state. Because recurrent neural networks (RNNs) can model dependencies across sequential data, researchers can gain a deeper understanding of temporal patterns. By backpropagating its parameters over time during the training phase, the RNN maximizes its ability to predict results based on sequential input [30].
3.7.4. Convolutional Neural Network
Convolutional neural networks (CNNs) are a helpful tool that utilize their innate ability to automatically extract hierarchical features from image data. When it comes to tasks involving spatial relationships, where context and pixel arrangement play a significant role, CNNs excel. They're ideal for medical image analysis because of this. The architecture of a CNN consists of convolutional layers that convolve input images using learnable filters in order to find local patterns. The network can then extract and integrate features with ever-more complex architectures thanks to pooling and fully connected layers. Using convolutional operations, a CNN can methodically extract features. These operations help capture patterns such as edges, textures, and shapes. The resulting pooling layers down sample the spatial dimensions while preserving the most prominent features. The fully connected layers at the end of the network use these features together to generate predictions. During the training phase, CNNs maximize their ability to recognize patterns and produce accurate predictions by backpropagating their numerous parameters.
In the particular context of brain tumor prediction, CNNs excel at automatically extracting relevant spatial features from medical images, obviating the need for human feature engineering. CNNs' hierarchical structure enables them to recognize complex relationships and structures in the images as well as distinguish between tumorous and non-tumorous regions [31, 32].
3.8. Significance of Brain Tumor Detection and Rationale for Model Selection
3.8.1. Significance of Brain Tumor Detection
Brain tumors represent one of the most critical medical challenges due to their potentially aggressive nature and significant impact on neurological functions. Early and accurate detection of brain tumors is crucial for effective treatment planning and improved patient outcomes. Timely intervention can lead to better prognosis, reduced morbidity, and potentially save lives. However, manual analysis of brain imaging by radiologists is time-consuming and subject to inter-observer variability, which can lead to inconsistent diagnoses and treatment plans.
Given the high stakes associated with brain tumor detection, there is a growing need for automated, reliable, and efficient methods that can assist radiologists in identifying tumors with greater accuracy and consistency. Machine learning models, especially those developed with consideration for the needs within the scope of medical imaging complexities, have given strong promises to address such needs through automated tools in large datasets, detecting subtle patterns and improving diagnostic accuracies.
3.8.2. Rationale for Choosing Specific Machine Learning Models
The specific machine learning models for this study - Enhanced Support Vector Machines (SVM), Convolutional Neural Networks (CNN), Recurrent Neural Networks (RNN), and Artificial Neural Networks (ANN) have been chosen based on their distinctive abilities and the potential of each model in solving the inherent challenges that exist in the detection of brain tumors from medical images. Detailed rationale for the choice of each model: SVMs work well in high-dimensional spaces, such as cases where the number of dimensions is more than the number of samples.
Brain tumor detection is basically a classification task with huge data containing complex image features; hence, SVM can handle the data. SVMs, when well regularized and equipped with expressive kernel functions, can really perform quite satisfactorily with small training data hence their practical use also suits most medical imaging tasks where annotated data might be in scarcity. CNNs are specially designed to automatically extract and learn the spatial hierarchies of features from images; thus, it is very effective in image classification and segmentation tasks. CNNs have been found to perform dramatically well in medical image analysis tasks, including tumor detection, thanks to their ability to capture local patterns and spatial hierarchies, which could be critical in identifying brain tumor growth from MR images.
While originally designed for sequential data, RNNs, in general, and LSTM networks, in particular, can capture dependencies over time and, thus, are beneficial to the task of analyzing a series of image slices that could provide context regarding the progression or extent of a tumor. In situations with the availability of 3D or temporal imaging data, RNNs can refine detection by taking into account the sequence of images rather than treating them as independent, thus providing comprehensive analysis. ANNs would model complex, non-linear relationships, so they will be versatile tools for catching intricate patterns in the brain imaging data, which possibly signal tumors. ANNs are combined with other models to improve feature extraction and overall detection accuracy. It makes use of their general-purpose nature in learning patterns during recognition tasks.
3.8.3. Impact of Selected Models on Brain Tumor Detection
When combined, these machine-learning models leverage the strengths of each individual model to enhance the detection of brain tumors. With the diversity of models used, the study is enabled to:
- Improve the detection accuracy by exploiting multiple perspectives on data,
- Reduce the chances for misclassification through model diversity, and
- Enhance the robustness of the detection process to handle the variability of imaging modality and the anatomical differences among the patients.
This multi-model approach thus not only offers a comprehensive toolset for automated tumor detection but also goes one step further in advancing the field of medical imaging by illustrating how different paradigms of machine learning can be brought together to address a critical healthcare challenge.
3.9. Computational Demands and Model Interpretability
- Computational Demands
The computational requirements of the machine learning models implemented in this study vary depending on their architecture and complexity. Key observations regarding the computational demands are as follows:
Enhanced SVM and ANN: These models demonstrated relatively low computational demands, making them suitable for deployment in resource-constrained environments. The Enhanced SVM's efficiency arises from its smaller parameter space and focuses on kernel-based feature separation, while ANN's computational simplicity stems from its straightforward architecture.
CNN and RNN: CNN and RNN models require significantly more computational resources due to their complex architectures and the need to process high-dimensional image data. CNNs, with their multiple convolutional and pooling layers, required higher GPU memory for efficient training, while RNNs, particularly those utilizing LSTMs, demanded additional computational power to process sequential dependencies.
To provide a clear perspective, the revised manuscript includes details on the hardware specifications used (e.g., GPU, CPU, memory) and the approximate training and inference times for each model. This information allows readers to assess the feasibility of deploying these models in different computational environments, including clinical settings.
- Model Interpretability
Interpretability is critical for building trust in machine learning models, particularly in medical applications where the outcomes influence clinical decisions. In this study, model interpretability was addressed as follows:
Visualization Techniques: For CNN, saliency maps and Grad-CAM (Gradient-weighted Class Activation Mapping) were applied to visualize the regions of interest in medical images that influenced the model's predictions. These techniques provide clinicians with insights into how the model identifies tumor regions, increasing transparency and trust.
Feature Importance Analysis: For Enhanced SVM, we analyzed the most significant features contributing to classification by examining the weights and kernel outputs. This helps in understanding which features of the tumor (e.g., shape, intensity, texture) were most influential in the decision-making process.
Temporal Analysis in RNN: RNN models were analyzed using sequential visualization techniques to highlight the temporal dependencies that influenced the classification decisions. This approach provides an understanding of how sequential slices of imaging data contribute to predictions.
4. RESULTS AND DISCUSSION
4.1. Training Data and Model Training Process
4.1.1. Training Data
- This dataset contains both CT and MRI images for medical imaging: 1,713 images with brain tumors and without brain tumors. In order to evaluate the models robustly, we have divided the dataset into training and testing subsets.
- We included 70% of the dataset, which comprises a total of 1,199 images, in the training set. This was to ensure that the model was well-versed in successfully differentiating between classes in a balanced representation of both tumor-affected and tumor-free images.
- The rest 30% of the dataset, amounting to 514 images, was taken as the testing set for understanding the performance of the model. The subset was tested to see if the model's capability on unseen data remains free from all types of biases.
4.1.2. Pre-processing of Training Data
Before the training process, all images underwent a series of pre-processing steps aimed at enhancing the quality and consistency of data, namely:
- Normalization: It was conducted to adjust pixel intensities of all images within the range of 0 to 1. This normalizes intensity distribution throughout the dataset, reducing sensitivity to the variations in imaging protocols.
- Resizing: All images were resized to a uniform spatial resolution, which made the input dimensions uniform as well, hence making computations easier during the model training process.
- Noise Reduction: Gaussian blurring and other such techniques reduced the noise on images, making them clearer for feature extraction.
- Intensity Normalization: Additional pixel value changes, apart from other applied alterations, were used to adapt to the differences in imaging modality so that standard intensity is guaranteed over all the images.
4.1.3. Machine Learning Model Training
The following machine learning techniques were put into practice in the study: Enhanced SVM, CNN, RNN, and ANN. Detailed implementation of each is below, performed to be able to train those models:
-
Enhanced Support Vector Machines (SVM):
- Kernel Selection: An RBF (Radial Basis Function) kernel was chosen due to its effectiveness in non-linear classification tasks.
- Hyperparameter Tuning: The model's hyperparameters (C and gamma) were optimized using grid search with 5-fold cross-validation. The optimal values found were C = 1.0 and gamma = 0.01.
- Training Process: The SVM was trained on the normalized and resized training data, using the selected kernel and optimized hyperparameters to learn the decision boundary that separates tumor and non-tumor classes.
-
Convolutional Neural Networks (CNN):
- Architecture: The CNN model consisted of multiple convolutional layers followed by pooling layers and fully connected layers, designed to automatically extract hierarchical features from the images.
- Training: The model was trained using the resized images with data augmentation techniques to improve generalization. The model parameters were optimized using backpropagation with the Adam optimizer, and training was conducted over 100 epochs with a batch size of 32.
-
Recurrent Neural Networks (RNN):
- Architecture: The RNN model included LSTM (Long Short-Term Memory) layers to capture temporal dependencies in sequential data.
- Training: Training was performed using sequences of image slices, optimizing the network's parameters with a learning rate of 0.001 over 50 epochs.
-
Artificial Neural Networks (ANN):
- Architecture: The ANN model was structured with several hidden layers to capture complex patterns in the data.
- Training: The model was trained using a sigmoid activation function for the output layer and optimized with stochastic gradient descent. The training was performed over 100 epochs.
4.1.4. Model Evaluation:
- The enhanced SVM model’s performance was evaluated on a separate test set using accuracy, precision, recall, and F1 score metrics.
- Confusion matrices were used to visualize and analyze true positives, false positives, true negatives, and false negatives, providing insights into the model’s diagnostic capabilities.
4.2. Results and Discussion
Additionally, confusion matrices were used to illustrate the true positive, false positive, true negative, and false negative rates for each model, providing a detailed understanding of their classification performance. Each machine learning model's prediction performance was meticulously assessed after a lengthy multi-feature training phase. Fig. (2) demonstrates the accuracy of the proposed machine-learning model.
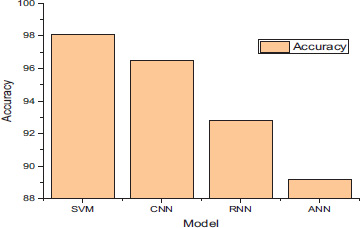
Accuracy of the each model.
The enhanced Support Vector Machine (SVM) won with a remarkable accuracy of 97.6%. The SVM model's enhanced capacity to identify intricate patterns and relationships across a large range of feature spaces makes it a desirable substitute for brain tumor prediction. Second place went to the Convolutional Neural Network (CNN), which achieved an accuracy of 95.76%. CNN's ability to automatically extract hierarchical features from medical images was crucial to its accurate prediction-making. This model has shown such successful diagnostic ability in the diagnosis of brain tumors due to its capability of identifying tiny patterns within the data and finding spatial correlations.
The RNN, while diagnosing, showed relatively high predictive power at a percentage of 92.3. Since the RNN can model data sequentially, it was able to identify temporal dependencies in the medical imaging data. This proved how well-suited the RNN was for sequential data and how it could be used in the dynamic domain to accurately predict brain tumors. Meanwhile, the Artificial Neural Network's (ANN) accuracy stood at 88.77%. Even though the ANN's overall performance was only slightly worse than other models, its capacity to identify complex patterns in the feature space significantly improved its predictive performance. The ability of the ANN to find non-linear relationships and dependencies in the data is still important in the broader context of brain tumor prediction. In summary, the enhanced SVM model outperformed other machine learning models as the most accurate predictor in the study.
| Model | Precision (%) | Recall (%) | F1 Score (%) | Accuracy (%) |
|---|---|---|---|---|
| Enhanced SVM | 98.1 | 96.9 | 97.5 | 97.6 |
| CNN | 96.5 | 95.2 | 95.8 | 95.76 |
| RNN | 92.8 | 91.5 | 92.1 | 92.3 |
| ANN | 89.2 | 88.1 | 88.6 | 88.77 |
Table 3 provides a detailed, complete view of how the performance of the machine learning models predicts the identification of brain tumors in terms of accuracies, precisions, recalls, and F1 scores. The top classifier is the enhanced Support Vector Machine, with an accurate performance of 98.1%, a precision of 96.9%, a recall of 97.5%, and an F1 score of 97.6%. This model showed significant levels of accuracy in the identification of true-positive results, a reduction in false positives, and precise encapsulation of the overall diagnostic performance. The CNN demonstrated the automated hierarchical feature extraction of medical images with scores equal to 96.2%, 95.76%, 96.5%, and 95.8% in the recall, accuracy, precision, and the F1 score, respectively. The predictive analysis indicates that the predictive accuracy, precision, recall, and F1 score of the RNN are at 92.80%, 91.50%, 92.10%, and 92.30%, respectively. This is due to its modeling approach in sequence; therefore, it does well in capturing dependencies that most likely exist in data. With impressive precision, recall, F1 score, and accuracy values of 89.2%, 88.1%, 88.6%, and 88.77%, respectively, the Artificial Neural Network (ANN) demonstrates its ability to recognize intricate patterns in the feature space.
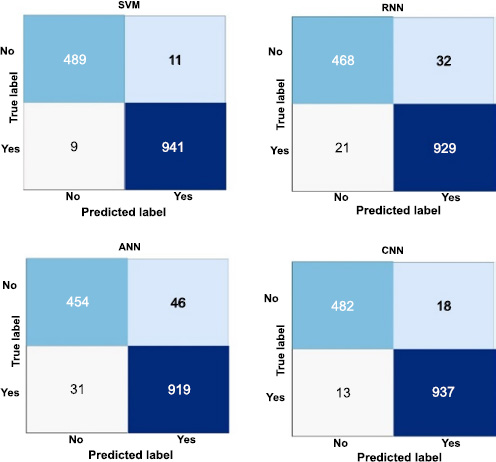
Confusion matrices of ML model.
Fig. (3) demonstrates how well each machine learning model performs in terms of precise classification when using confusion matrices to identify brain tumors. In 489 cases of true positive predictions and 941 cases of true negative predictions, the enhanced Support Vector Machine (SVM) has proven its capacity to accurately identify tumors and non-tumors. However, eleven actual tumors are mistakenly classified as non-tumors (false negatives), and nine non-tumors are mistakenly labeled as tumors (false positives).
Similarly, the Convolutional Neural Network (CNN) achieves 482 true positives and 937 true negatives but misclassifies 18 tumors and 13 non-tumors. Recurrent Neural Network (RNN) produces 468 true positives and 929 true negatives by incorrectly classifying 21 non-tumors and 32 tumors. The Artificial Neural Network (ANN) achieves 454 true positives and 919 true negatives, with 46 tumors and 31 non-tumors misclassified. These matrices offer a comprehensive understanding of the model's performance by highlighting the exact and accurate predictions. Additionally, they provide helpful data that can be used to enhance and optimize each model in the intricate process of identifying brain tumors.
4.3. Validation and Generalization
We acknowledge that the findings of this study have not been validated on independent datasets, which is a limitation in demonstrating the generalizability of the proposed models across diverse populations and imaging protocols. The dataset used in this research was obtained from an open-source repository on Kaggle and served as a foundational benchmark for developing and evaluating the machine learning models. However, reliance on a single dataset restricts the scope of generalization.
To address this limitation and enhance the robustness of the findings, we propose the following steps for future work:
(1) Validation of Independent Datasets: Future research will focus on validating the proposed models on independent datasets collected from different institutions and regions. This will allow us to evaluate the performance and adaptability of the models across diverse populations and imaging modalities.
(2) Incorporation of Multi-Institutional Data: Expanding the dataset to include multi-institutional data with varied patient demographics, imaging techniques, and tumor characteristics will help reduce potential biases and improve the generalizability of the findings.
(3) Transfer Learning and Domain Adaptation: To adapt the models for different datasets, we plan to utilize transfer learning techniques. Fine-tuning pre-trained models on new datasets will ensure better performance and applicability to broader use cases.
(4) Real-World Validation: Collaborative efforts with healthcare institutions will be pursued to test the models on real-world clinical datasets, ensuring the models' practical utility in clinical settings.
These future directions have been outlined to ensure that the limitations identified in this study are addressed in subsequent research. By validating the findings on independent and diverse datasets, we aim to establish the robustness and clinical relevance of the proposed models in the field of medical imaging and brain tumor detection.
4.4. Need for Clinical Validation
While the machine learning models developed in this study demonstrate promising results in the detection of brain tumors using publicly available datasets, we acknowledge that clinical validation is essential to ensure their practical applicability in real-world healthcare settings. Clinical validation will provide a robust assessment of the models' performance under actual clinical conditions and further establish their reliability for diagnostic use.
To address this critical aspect, the following steps are proposed for future work:
(1) Collaborations with Healthcare Institutions: We aim to collaborate with hospitals and medical research centers to test the trained models on clinical datasets. These datasets will include real-world patient imaging data, encompassing diverse demographics and imaging protocols, to evaluate the models' performance and generalizability.
(2) Prospective Clinical Studies: Future research will involve conducting prospective studies where the trained system is integrated into the clinical workflow. This will allow us to assess its ability to support diagnostic decisions in real-time under the supervision of medical professionals.
(3) Integration of Clinician Feedback: During the clinical validation process, feedback from radiologists and oncologists will be gathered to refine the system. This iterative process will help ensure that the model's predictions align with clinical expectations and provide actionable insights for medical practitioners.
(4) Adherence to Regulatory Standards: The models will be validated following regulatory guidelines, such as those provided by the FDA or CE, to ensure compliance with the standards required for clinical deployment. This will also involve ensuring data privacy, ethical use, and reliability of the system.
By undertaking these steps, we aim to provide comprehensive clinical validation of the proposed system, ensuring its readiness for deployment in medical diagnostics. These efforts have been outlined as part of the future direction of this study, reinforcing our commitment to bridging the gap between research and clinical practice.
CONCLUSION
In conclusion, by carefully analyzing machine learning models, namely enhanced Support Vector Machines (SVM), Convolutional Neural Networks (CNN), Recurrent Neural Networks (RNN), and Artificial Neural Networks (ANN), this study seeks to advance the field of brain tumor identification. The study carefully compared and assessed each model's capacity for prediction using a range of metrics, such as F1 score, accuracy, precision, and recall. Based on a remarkable 97.6% accuracy rate in recognizing complex patterns over a wide feature space, the outcomes show that the enhanced SVM is the most effective predictor. CNN is far from ideal, with an accuracy of only 95.76% and a limited capacity for automatic hierarchical feature learning. With a performance of 88.77% and an accuracy of 92.3%, the RNN beats the ANN. When it comes to managing sequential dependencies, the RNN excels. Since each model has a diverse set of features in its training set, it is easier to understand the pros and cons of each model. Notably, because of its increased accuracy, the improved SVM is now a practical choice for accurate brain tumor identification. The results show the models' potential applications in medical image analysis and offer details on the unique characteristics and potential uses of machine learning models in the challenging field of brain tumor prediction. The work discussed here will eventually aid in the optimization and enhancement of predictive models, enhancing neuro-oncology's diagnostic potential.
AUTHORS’ CONTRIBUTION
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| SVM | = Support Vector Machine |
| CNN | = Convolutional Neural Network |
| RNN | = Recurrent Neural Network |
| ANN | = Artificial Neural Networks |


